Smarter decisions at point of care
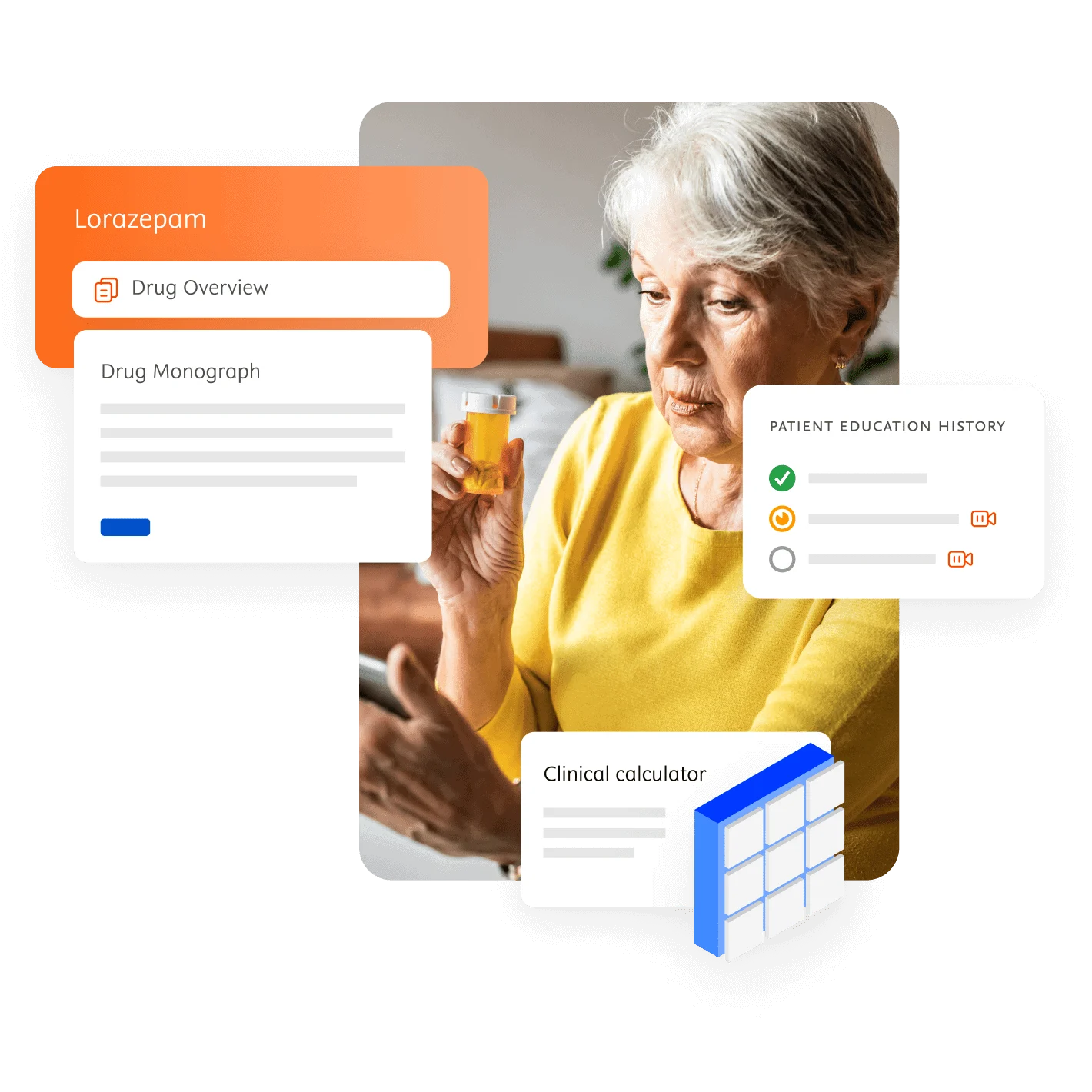
Inform medication decisions
Enhance patient care and drug safety
Improve drug therapy outcomes
Manage drug costs
Empower smart clinical and business decisions

Unfortunately we don't fully support your browser. If you have the option to, please upgrade to a newer version or use Mozilla Firefox, Microsoft Edge, Google Chrome, or Safari 14 or newer. If you are unable to, and need support, please send us your feedback.
We'd appreciate your feedback.Tell us what you think!(opens in new tab/window)
Drug Information Solutions
Using today’s technology and evidence-based content, your healthcare professionals can help solve daily medication challenges.
Inform medication decisions
Enhance patient care and drug safety
Improve drug therapy outcomes
Manage drug costs
Empower smart clinical and business decisions

Leverage the power of daily data delivery for quick access to trusted content
Enhance IT and operational value for customers
Solve daily medication challenges
Track and analyze drug data
Ensure regulatory compliance
Boost workflow efficiency
Optimize operational and financial performance

Integrated drug database and clinical support engine
Improve medication decisions with trusted knowledge
Data-science derived drug acquisition cost to support business analytics
